Echographie pulmonaire et évaluation de la surcharge hydrique en dialyse péritonéale
DOI :
https://doi.org/10.25796/bdd.v8i3.87083Mots-clés :
lignes-B, échographie publmonaire, dialyse péritonéale, hypervolémie subclinique, hydratationRésumé
La gestion des fluides reste un défi majeur dans la dialyse péritonéale (DP), en particulier en raison de la difficulté à détecter l’hypervolémie subclinique. Cette étude transversale, menée dans un seul centre sur un échantillon de 22 patients ; nous avons examiné l’utilité de l’échographie pulmonaire (LUS) comme outil complémentaire pour l’évaluation de l’état volumique. À l’aide d’un protocole de balayage en 8 zones et d’un système de cotation des lignes B, l’échographie pulmonaire a été réalisée lors de visites de routine, parallèlement à l’évaluation de la veine cave inférieure (VCI) et à l’analyse par bioimpédance électrique (BIA). Les résultats ont montré une forte corrélation entre les scores des lignes B et l’hyperhydratation mesurée par la BIA (r = 0,625) et l’indice de collapsibilité de la VCI (r = –0,722). Il est à noter que la moitié des patients considérés comme cliniquement euvolémiques présentaient des signes d’hypervolémie selon les critères échographiques. Ces résultats suggèrent que la LUS est une méthode non invasive, pratique et efficace pour identifier une surcharge liquidienne non évidente lors d’une évaluation clinique standard, ce qui justifie son intégration dans les soins de routine des patients sous DP.
INTRODUCTION
L'estimation du statut volumique idéal est un défi constant en néphrologie. Elle repose généralement sur une évaluation clinique associée à d'autres outils diagnostiques tels que les peptides natriurétiques et la spectroscopie par bioimpédance (BIS). Plusieurs méthodes ont été mises au point pour évaluer avec précision l'état hydrique des patients dialysés, notamment la BIS, la mesure du diamètre de la veine cave inférieure (VCI) par échographie, l'analyse de biomarqueurs tels que les peptides natriurétiques et, plus récemment, l'observation de comètes pulmonaires à l'aide d'une échographie thoracique 1.
En 1996, le Dr Daniel Lichtenstein a décrit pour la première fois l'artefact en queue de comète détecté lors d'une échographie pulmonaire chez une cohorte de patients présentant aussi bien une oedème pulmonaire cardiogénique que non cardiogénique 2 . Cet artefact est causé par des micro-réflexions à l'interface sous-pleurale, que l'échographie interprète comme une distance, due à la présence d'eau pulmonaire extravasculaire ou de petites interfaces air-liquide, ce qui se traduit par un rayon étroit, semblable à un rayon laser, sur l'écran. Cet artefact, désormais connu sous le nom de « ligne B », a contribué à améliorer notre capacité à détecter les oedèmes pulmonaires cliniques et, en particulier, subcliniques, offrant une alternative plus précise et plus accessible aux méthodes traditionnelles telles que l'auscultation pulmonaire ou la radiographie thoracique 3 . L'une de ses principales limites est son manque de spécificité. Les lignes B sont un signe échographique du syndrome interstitiel pulmonaire, mais elles ne permettent pas de différencier l'épaississement des septa interlobulaires causé par un excès d'eau pulmonaire de l'épaississement fibrotique, comme on le voit dans la fibrose pulmonaire (lignes B sèches). Il convient donc d'interpréter avec prudence les résultats de la LUS chez les patients atteints ou suspectés de fibrose pulmonaire 4.
De nombreuses études ont démontré l'utilité clinique de l'échographie pulmonaire (lung Ultrasonography (LUS)). Des études telles que LUS-HF et CLUSTER-HF ont démontré l'utilité de la LUS pour orienter un traitement diurétique plus intensif chez les patients atteints d'insuffisance cardiaque, ce qui a permis de réduire les lignes B et d'améliorer la prise en charge de la surcharge liquidienne 56 . Bien qu'il existe peu d'études sur l'hypervolémie subclinique chez les patients atteints d'insuffisance rénale chronique (IRC), une étude menée par Baki et al. a démontré que l'association d'une élévation des taux de peptide natriurétique de type B et d'une diminution de l'indice de collapsibilité de la veine cave inférieure (IVCI) augmentait la spécificité et la valeur prédictive positive pour la détection de l'hypervolémie subclinique 7.
Il est bien connu que la prévalence de l’hypervolémie chez les patients en DP est considérable et associée à une augmentation de la mortalité 8. Une surcharge volumique persistante augmente la rigidité artérielle, entraîne une augmentation de la pression artérielle systolique, une diminution de la pression artérielle diastolique et une augmentation de la pression différentielle. Cela augmente la postcharge du ventricule gauche, entraînant une hypertrophie ventriculaire gauche (HVG). Tous ces effets ont été identifiés comme des facteurs de risque indépendants de morbidité et de mortalité cardiovasculaires dans les maladies rénales terminales (MRT) 9 10.
Une congestion pulmonaire asymptomatique a été décrite chez une proportion significative de patients dialysés 11. Dans une étude multicentrique menée par Zoccali et al., une congestion pulmonaire modérée à sévère était présente chez 45 % des patients hémodialysés, 71 % d’entre eux étant asymptomatiques ou ne présentant que de légers symptômes d’insuffisance cardiaque 12. Une autre étude menée par Enia et al. a rapporté que 58 % des patients hémodialysés présentaient une congestion pulmonaire modérée à sévère, dont 38 % étaient asymptomatiques (classe NYHA I) 13. De plus, Mallamaci et al. ont démontré que 57 % des patients dialysés asymptomatiques présentaient une congestion pulmonaire modérée à sévère 14. Ainsi, certains patients dialysés sont considérés à tort comme euvolémiques.
Une plus grande précision dans l’évaluation de la volémie est un besoin important non satisfait chez les patients dialysés, en particulier ceux sous DP. Certaines études suggèrent que les patients sous DP pourraient être plus sujets à l’hyperhydratation que ceux sous hémodialyse (HD). Il est particulièrement difficile d’atteindre l’éuvolemie chez les patients sous DP, car cette thérapie repose sur l’autotraitement, l’ultrafiltration quotidienne et la fonction rénale résiduelle, qui peuvent varier considérablement d’un individu à l’autre [8,15,16].
La LUS a été validée comme une méthode efficace pour évaluer l'eau extracellulaire pulmonaire, en particulier chez les patients atteints d'insuffisance cardiaque et sous hémodialyse 116. Bien que son rôle chez les patients sous DP reste incertain, elle peut jouer un rôle important dans l'évaluation de l'état volémique chez ces patients. En hémodialyse, la LUS a démontré une forte valeur pronostique, car le nombre de lignes B est corrélé aux événements cardiovasculaires indésirables et à la mortalité, et l'ultrafiltration guidée par la LUS permet de réduire en toute sécurité la congestion pulmonaire sans augmenter les complications intradialytiques. En dialyse péritonéale, les résultats de la LUS ne sont pas toujours corrélés à la bioimpédance ou aux signes cliniques. Cela suggère que la LUS et d'autres méthodes d'évaluation évaluent différents compartiments liquidiens et sont donc complémentaires 1111517.
La technique elle-même est rentable et largement accessible, ne nécessitant que 3 à 5 minutes pour être réalisée. Elle peut être effectuée efficacement par des techniciens formés et présente une faible variabilité inter-observateur 18. Le système de notation en 8 points a montré d’excellentes performances diagnostiques dans l’évaluation des patients atteints d’IRT 1920. La mesure de l’IVCI ajoute des informations sur l’état de la volémie intravasculaire 21.
Compte tenu de ces considérations, la combinaison de différentes méthodes et technologies pourrait améliorer la détection de l'hypervolémie subclinique chez les patients en DP. Nous avons donc cherché à estimer sa prévalence à l'aide de la LUS et à déterminer la concordance entre les résultats de la LUS, l'IVCCI et d'autres paramètres et outils cliniques, tels que le BIS.
METHODES
Cette étude a été approuvée par le comité d'éthique local. Tous les participants ont donné leur consentement éclairé.
Sélection et recrutement des patients
Il s'agissait d'une étude transversale opportuniste menée auprès de patients sous dialyse péritonéale (DP) lors de consultations cliniques de routine. Les patients n'ont pas été présélectionnés en fonction de caractéristiques spécifiques, mais ont été inclus quand ils répondaient aux critères suivants : durée de la DP supérieure à 3 mois, absence d'infections actives et volonté de participer. Le recrutement a eu lieu lors de consultations ambulatoires de routine au cours du premier semestre 2023 et dépendait de la disponibilité du personnel et de l'équipement nécessaires pour effectuer les évaluations. Tous les patients éligibles qui se sont présentés pendant la période d'étude et pour lesquels les conditions logistiques le permettaient (c'est-à-dire la disponibilité des ressources humaines et techniques) ont été invités à participer. Il n'y a pas eu de processus d'inclusion subjectif ou sélectif ; les patients ont plutôt été recrutés de manière consécutive, lorsque les conditions le permettaient.
Données démographiques
Les données relatives à l'âge, au sexe, à la cause de l'IRC, aux antécédents médicaux de tabagisme, aux comorbidités telles que le diabète, l'insuffisance cardiaque ou les maladies pulmonaires ont été extraites des dossiers médicaux des patients.
Évaluation clinique
Lors de la consultation, les patients ont été interrogés sur les symptômes d'hypervolémie : orthopnée, dyspnée. Un examen physique a été réalisé et les données relatives au poids, à la pression artérielle, à la présence de crépitements à l'auscultation pulmonaire et à la présence d'oedème prétibial ont été notées.
Données de laboratoire
Les paramètres biologiques mesurés lors de la dernière consultation en DP ont été consultés. Les dernières valeurs sériques du NT-proBNP, du sodium, du chlorure, du bicarbonate et de l'albumine ont été enregistrées.
Examens d’imagerie
La plupart des patients avaient subi une radiographie thoracique et une échocardiographie au cours de l'année écoulée. La présence ou l'absence d'épanchement pleural à la radiographie thoracique a été notée. Les données échographiques concernant le diamètre de l'oreillette gauche, la fraction d'éjection ventriculaire gauche, l'indice de masse ventriculaire gauche et la présence/absence d'épanchement péricardique ont été recueillies.
Analyse de bio-impédance
Une analyse de bio-impédance avec un moniteur de composition corporelle (Fresenius Medical Care) a été réalisée systématiquement lors de chaque consultation de DP. Les dernières données d'analyse de bio-impédance concernant l'état d'hydratation ont été enregistrées pour chaque patient le jour même.
Évaluation échographique
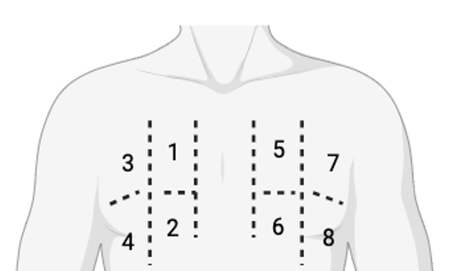
Nous avons réalisé une échographie lors des consultations de DP de routine, en évaluant la LUS à l’aide d’un protocole de balayage semi-quantitatif en 8 sites. Quatre sites dans chaque hémithorax ont été balayés (Figure 1). Chaque site (1 à 8) a été noté selon le Tableau I Le score des lignes B et le nombre total de lignes B pour chaque site ont été enregistrés.
Figure 1. Protocole de balayage en 8 sites. Chaque hémithorax est divisé verticalement par la ligne médio-claviculaire et horizontalement par le 3e espace intercostal.
| Modèle échographique pulmonaire | Score |
|---|---|
| Normal Motif en ligne A ou < 3 lignes/espace | 0 |
| Lignes B bien séparées (≥ 3 par espace intercostal) | 1 |
| Lignes B coalescentes (≥3 par espace intercostal) | 2 |
| >10 lignes B/consolidation | 3 |
L’évaluation de la veine cave inférieure (VCI) a été réalisée lors des visites de routine afin de calculer l’indice de collapsibilité de la VCI (IVCCI). L’IVCCI a été défini comme suit (Figure 2) :
Figure 2.Calcul de indice de collapsibilité de la veine cave inférieure (IVCCI).
Formation des opérateurs et reproductibilité interobservateurs
Les échographies pulmonaires et les évaluations de la VCI ont été réalisées par deux opérateurs ayant suivi une formation dédiée à l’échographie au chevet du patient (POCUS) pour les néphrologues et ayant participé à un stage pratique d’une semaine dans un centre reconnu, où des évaluations régulières des lignes B et des mesures de la VCI sont effectuées. Cette formation visait à garantir la cohérence et la fiabilité des évaluations échographiques.
Définition de l’hypervolémie subclinique
En l’absence de critères standardisés pour diagnostiquer l’hypervolémie subclinique dans la littérature médicale, nous avons adopté une approche échographique exploratoire combinant les résultats du LUS et l’IVCCI. L’hypervolémie subclinique a été définie comme l’absence de signes cliniques manifestes de surcharge liquidienne associée à un score total des lignes B ≥ 4 et/ou un IVCCI < 50 %. Si les lignes B sont des marqueurs établis de l’eau pulmonaire extravasculaire et que l’IVCCI < 50 % suggère une surcharge volumique, les seuils spécifiques utilisés ici ne sont pas encore normalisés ni validés de manière universelle. Cette combinaison de paramètres échographiques vise à améliorer la précision du diagnostic, mais doit être considérée comme une approche pragmatique et génératrice d’hypothèses en attendant une validation plus approfondie.
Analyse statistique
Les variables catégorielles sont présentées sous forme de fréquences et de pourcentages, et les variables continues sous forme de moyennes et d’écarts-types, ou de médianes et d’intervalles interquartiles pour les variables présentant des distributions asymétriques. Les distributions normales ont été vérifiées en évaluant l’asymétrie et l’aplatissement. Toutes les valeurs p rapportées sont bilatérales, et une valeur p de 0,05 indique une signification statistique. L’analyse de corrélation a été réalisée à l’aide du test de corrélation de Pearson, avec les intervalles de confiance (IC) à 95 % correspondants. Les analyses ont été effectuées à l’aide du logiciel SPSS Statistics, version 25.
RESULTATS
Vingt-deux patients ont été inclus. L’âge moyen était de 61 ± 11 ans, 59 % étaient des hommes. La durée médiane de la DP était de 30 mois (Tableau II). La prévalence du diabète sucré était similaire dans les deux groupes. En ce qui concerne les variables liées à la technique, 27 % de la cohorte totale était sous dialyse péritonéale automatisée (DPA), avec une proportion légèrement plus élevée (40 %) dans le groupe à B-line plus élevée. Il est à noter que seuls 5 patients avaient un score B-line ≥ 4.
La fonction rénale résiduelle (FRR) avait tendance à être plus faible chez les patients présentant des scores B-line plus élevés (moyenne de 1334 ± 887 ml dans la cohorte totale contre 1000 ml dans le groupe présentant les scores B-line les plus élevés). Les patients présentant des scores B-line plus élevés avaient également une pression artérielle diastolique moyenne significativement plus élevée (75 ± 11 vs 89 ± 15 mmHg, p = 0,029) et des taux sériques d’albumine plus faibles (4,1 ± 0,3 vs 3,7 ± 0,5 g/dL, p = 0,032), comme le détaille le Tableau III.
| Total (n = 22) |
Score < 4 (n = 17) |
Score ≥4 (n=5) |
Valeur p | |
|---|---|---|---|---|
| Âge – moyenne ± écart-type | 61 ± 11 | 60 ± 12 | 57 ± 4 | 0,25 |
| Homme – n (%) | 13 (59) | 9 (53) | 4 (80) | 0,360 |
| Comorbidités – n (%) | ||||
| Insuffisance cardiaque | 4 (18) | 4 (24) | 0 | / |
| Diabète sucré | 5 (23) | 4 (24) | 1 (20) | 0,687 |
| Antécédents tabagiques | 4 (18) | 3 (21) | 1 (20) | 0,489 |
| Maladie pulmonaire | 2 (9) | 2 (11) | 0 | / |
| Variables liées à la technique | ||||
| Durée de la maladie (mois) – médiane (Q3-Q1) | 30 (53-12) | 34 (55-18) | 12 (31-4) | 0,085 |
| DP automatisée – n (%) | 6 (27) | 4 (24) | 2 (40) | 0,535 |
| Utilisation d'icodextrine – n (%) | 16 (72) | 12 (71) | 4 (80) | 1 |
| RRF – moyenne ± écart-type | 1334 ± 887 | 1300 (1600-1050) | 1000 (1650-100) | 0,233 |
| Ultrafiltration – moyenne ± écart-type | 711 ± 513 | 615 ± 413 | 1086 ± 697 | 0,07 |
| Total (n = 22) |
Score < 4 (n = 17) |
Score ≥4 (n=5) |
Valeur p | |
|---|---|---|---|---|
| Données cliniques/Examen physique | ||||
| Dyspnée (oui) – n (%) | 2 | 1 (6) | 1 (20) | 0,411 |
| Craquements à l'auscultation pulmonaire (oui) – n (%) | 3 (14) | 2 (12) | 1 (20) | 0,700 |
| Œdème périphérique (oui) – n (%) | 3 (14) | 3 (14) | 0 | / |
| TA systolique – (mmHg) moyenne ± ET | 135 ± 2 | 132 ± 20 | 143 ± 29 | 0,347 |
| BP diastolique – (mmHg) moyenne ± ET | 78 ± 13 | 75 ± 11 | 89 ± 15 | 0,029 |
| Résultats de laboratoire | ||||
| NT-ProBNP (pg/ml) – médiane (Q3-Q1) | 1143 (5323-668) | 861 (4243-649) | 4141 (16513-980) | 0,225 |
| Albumine sérique (g/dL) – moyenne ± ET | 4 ± 0,4 | 4,1 ± 0,3 | 3,7 ± 0,5 | 0,032 |
| Sodium sérique (mmol/l) – moyenne ± ET | 138 ± 3 | 138 ± 3 | 139 ± 2 | 0,677 |
| Cl sérique (mmol/l) – moyenne ± ET | 98 ± 4 | 99 ± 4 | 98 ± 2 | 0,791 |
| HCO3 sérique (mmol/l) – moyenne ± ET | 34 ± 3 | 24 ± 4 | 24 ± 4 | 0,581 |
| Spectroscopie par bioimpédance | ||||
| Surhydratation (L) – moyenne ± ET | 0,8 ± 1,5 | 0,3 ± 1,3 | 2,3 ± 0,9 | 0,005 |
| Évaluation de la veine cave inférieure | ||||
| VCI dMax (mm) – moyenne ± ET | 14 ± 13 | 12 ± 4 | 15 ± 3 | 0,139 |
| VCI dMin (mm) – moyenne ± ET | 7 ± 4 | 8 ± 3 | 12 ± 5 | 0,066 |
| IVCI (%) – moyenne ± ET | 40 ± 13 | 42 ± 12 | 31 ± 12 | 0,104 |
| Radiographie | ||||
| Épanchement pleural – n (%) | 6 (27) | 4 (23) | 2 (40) | 0,585 |
| Échocardiographie | ||||
| Épanchement péricardique – n (%) | 1 (5) | 0 | 1 (20) | / |
| FEVG – moyenne ± ET | 57 ± 9 | 56 ± 10 | 58 ± 7 | 0,681 |
| Diamètre de l'oreillette gauche (mm) – moyenne ± écart-type | 40 ± 6 | 40 ± 6 | 41 ± 6 | 0,685 |
| IMVI g/m2 – médiane (Q3-Q1) | 97 (122-80) | 100 (121-80) | 94 (140-74) | 0,845 |
| Médicaments | ||||
| Diurétiques – n (%) | 18 (82) | 13 (77) | 5 (100) | 0,585 |
| Nombre de médicaments anti-HTA – médiane (Q3-Q1) | 3 (3-1) | 3 (3-1) | 2 (4-1) | 0,869 |
Les patients présentant des scores de ligne B plus élevés avaient tendance à présenter un état d’hydratation élevé, tel qu’évalué par la BIA, et un IVCCI plus faible. Les résultats échocardiographiques n’ont révélé aucune différence significative entre les patients présentant des scores de ligne B élevés et faibles. Les paramètres clés, tels que la fraction d’éjection ventriculaire gauche (FEVG), ont été préservés dans tous les groupes, avec des valeurs toujours comprises dans la fourchette normale. Aucune variation notable de la taille des cavités cardiaques ou de la fonction valvulaire n’a été observée entre les groupes. De plus, les résultats radiographiques ont révélé que les patients présentant un score de ligne B plus élevé avaient une prévalence plus élevée de congestion pulmonaire, avec des radiographies thoraciques montrant des signes d’œdème interstitiel et de légers épanchements pleuraux.
Tous les patients du groupe présentant un score B-line élevé étaient sous diurétiques, avaient des taux d’ultrafiltration plus élevés et une fonction rénale résiduelle plus faible, ce qui souligne encore davantage la difficulté de la gestion des fluides.
Concordance entre les méthodes d’évaluation de la surcharge liquidienne
Nous avons observé une corrélation positive entre le score des lignes B et l’hyperhydratation sur la BIA (r = 0,625, p = 0,002). Des corrélations inverses entre le score des lignes B et l’IVCCI (r = -0,722, p = 0,001) et la fonction rénale résiduelle (r = -0,628 ; p = 0,002) ont été observées. Nous n’avons pas identifié de corrélation statistiquement significative entre le score des lignes B et le peptide natriurétique cérébral N-terminal (NT-proBNP) (r = 0,334, p = 0,129). Ces résultats sont résumés dans le Tableau IV. Des résultats similaires ont été obtenus en utilisant le nombre total de lignes B (par opposition au système de notation).
| Paramètre 1 | Paramètre 2 | Corrélation (r) | IC à 95 % [LL, UL] | Valeur p |
|---|---|---|---|---|
| Score B-line | Hyperhydratation (BIA) | 0,625 | [0,276, 0,828] | 0,002 |
| Score B-line | IVCCI | -0,722 | [-0,893, -0,37] | 0,001 |
| Score B-line | RRF | -0,628 | [-0,83, -0,281] | 0,002 |
| Score B-line | NT-proBNP | 0,334 | [-0,102, 0,662] | 0,129 |
Prévalence de l’hypervolémie subclinique chez les patients sous dialyse péritonéale
Nous avons réalisé une sous-analyse afin d’identifier la prévalence de l’hypervolémie subclinique chez les patients sous DP. Six patients (27 %) présentant des signes et symptômes manifestes d’hypervolémie, notamment une dyspnée, un œdème périphérique et des crépitements à l’auscultation pulmonaire, ont été exclus de cette évaluation. Huit patients (50 %) ont été classés comme présentant une hypervolémie subclinique, sur la base de critères échographiques, définis par un score B-line supérieur à 4 et/ou un IVCCI inférieur à 50 %.
Les patients diagnostiqués avec une hypervolémie subclinique présentaient un niveau moyen d’hyperhydratation significativement plus élevé (1,45 ± 1,2 L) que ceux sans hypervolémie subclinique (-0,37 ± 0,83 L ; p = 0,008), indiquant un volume élevé de rétention liquidienne. Ces patients présentaient également des taux sériques de NT-proBNP notablement élevés (p = 0,046), un biomarqueur couramment associé à une surcharge liquidienne et à un stress cardiaque. De plus, leur fonction rénale résiduelle (FRR) était significativement plus faible (p = 0,028), reflétant les difficultés potentielles d’élimination des liquides chez ce groupe de patients sous DP.
Malgré ces résultats, il n'y avait pas de différences significatives entre les groupes en ce qui concerne les autres paramètres démographiques et biologiques.
Le Tableau V résume les principales différences entre les patients présentant une hypervolémie subclinique et ceux présentant une normovolémie.
.
|
Hypervolémie subclinique (n = 8) |
Normovolémie (n=8) |
Valeur P |
|
|---|---|---|---|
| Homme – n (%) | 5 (62,5) | 4 (50) | 0,614 |
| Âge (années) – moyenne ± écart-type | 63,4 ± 9,9 | 58,7 ± 12,9 | 0,428 |
| Durée de la MP (mois) – moyenne ± écart-type | 54,88 ± 87,5 | 31 ± 24,44 | 0,798 |
| TA systolique (mmHg) – moyenne ± ET | 140 ± 19,9 | 127,4 ± 20,2 | 0,229 |
| TA diastolique (mmHg) - moyenne ± ET | 79,4 ± 9,4 | 71,5 ± 14,2 | 0,212 |
| Hyperhydratation (L) – moyenne ± écart-type | 1,45 ± 1,2 | -0,37 ± 0,83 | 0,008 |
| Sodium (mmol/l) – moyenne ± écart-type | 138,9 ± 1,8 | 137,9 ± 3,2 | 0,511 |
| Albumine g/dL – moyenne ± écart-type | 3,85 ± 0,46 | 4,05 ± 0,34 | 0,338 |
| NT-proBNP (pg/ml) – moyenne ± écart-type | 7132,5 ± 7588,1 | 1990,4 ± 2788,2 | 0,046 |
| Fonction rénale résiduelle (ml) – moyenne ± ET | 950 ± 594,62 | 1487,5 ± 418,1 | 0,028 |
DISCUSSION
Dans cette étude, la LUS s’est révélée être un outil précieux pour détecter l’hypervolémie subclinique chez les patients en DP. Des scores B-line plus élevés (≥ 4) étaient associés à un état d’hydratation accru, à des taux élevés de NT-proBNP et à une fonction rénale résiduelle réduite, malgré le fait que tous les patients étaient sous diurétiques. Ces résultats soulignent la nécessité de stratégies de gestion des fluides plus agressives, telles que l’icodextrine et les solutions hypertoniques, chez les patients présentant une résistance aux diurétiques et une diminution de la FRR.
Les fortes corrélations entre les scores de la ligne B et l’hyperhydratation détectée par BIA, ainsi que l’IVCCI, renforcent la sensibilité de la LUS dans la détection de la surcharge liquidienne. La LUS et la BIA fournissent des informations complémentaires : la LUS évalue l’eau pulmonaire extravasculaire, tandis que la BIA évalue l’eau totale du corps, l’eau extracellulaire (ECW) et l’excès d’eau intracellulaire 16. Compte tenu de sa nature non invasive et de son applicabilité au chevet du patient, la LUS est particulièrement bénéfique pour les patients présentant une fonction cardiaque limite, des épisodes récurrents d’insuffisance cardiaque ou une augmentation de l’ECW due à une hypoalbuminémie.
Cependant, une limitation importante de la LUS est la spécificité des lignes B, en particulier chez les patients atteints de maladies pulmonaires chroniques où des lignes B « sèches » peuvent apparaître indépendamment d’une surcharge liquidienne, ce qui peut brouiller l’interprétation 4. Dans notre cohorte, aucun des patients présentant un score de lignes B ≥ 4 ne souffrait d’une maladie pulmonaire chronique sous-jacente, ce qui a réduit ce facteur de confusion dans nos résultats. Néanmoins, une corrélation clinique prudente reste essentielle lors de l’application de la LUS dans des populations présentant des comorbidités pulmonaires.
Bien que notre étude n’ait pas été conçue pour établir des comparaisons directes entre la LUS et d’autres méthodes, les résultats soutiennent l’intégration des évaluations du score des lignes B aux côtés de la BIA et du NT-proBNP. La corrélation inverse entre les scores des lignes B et l’IVCCI (r = -0,722) confirme en outre la relation entre la congestion pulmonaire et la réduction de la collapsibilité veineuse. À l’inverse, la corrélation faible et non significative entre les scores B-line et le NT-proBNP (r = 0,334, p = 0,129) souligne la valeur discriminante limitée du NT-proBNP seul dans ce contexte, probablement en raison de multiples facteurs d’influence, notamment la fonction rénale résiduelle et les comorbidités. Par conséquent, le NT-proBNP doit être interprété avec prudence et de préférence en association avec d’autres outils d’évaluation 2223.
La pertinence clinique de ces résultats réside dans l’identification précoce de la surcharge liquidienne, en particulier chez les patients présentant une FRR en déclin (r = -0,628). La LUS peut fournir des informations en temps réel, améliorant ainsi la prise de conscience des patients et leur adhésion aux stratégies de gestion des fluides. La détection d’une hypervolémie subclinique pourrait impliquer des changements proactifs dans la pratique clinique, tels que l’ajustement des taux d’ultrafiltration, l’initiation ou l’intensification de l’utilisation de l’icodextrine, l’optimisation du traitement diurétique ou le renforcement de l’éducation des patients afin d’améliorer le respect des restrictions hydriques. Bien que certains ajustements thérapeutiques aient été apportés sur la base des résultats, ces interventions n’ont pas été standardisées et n’ont donc pas été analysées de manière systématique ni incluses dans cette étude. Les recherches futures devraient se concentrer sur l’implémentation de modifications de protocoles du traitement guidées par une approche holistique, qui devrait inclure les résultats de la LUS, afin de mieux évaluer leur impact sur la survie rénale, les résultats globaux et l’utilisation des soins de santé chez les patients en DP.
Il est important de noter que cette étude est la première à évaluer l’hypervolémie subclinique chez les patients en DP à l’aide de la LUS, soulignant sa faisabilité, sa courbe d’apprentissage courte et sa reproductibilité élevée. Si un appareil à ultrasons est disponible dans l’unité, son utilisation devrait être maximisée, car les protocoles LUS sont simples et faciles à mettre en œuvre dans la pratique courante.
Malgré ses avantages, notre étude présente certaines limites. La petite taille de l’échantillon (n = 22) réduit la puissance statistique et la généralisation des résultats. En outre, les différences entre les pratiques cliniques des différents centres et les biais de sélection potentiels doivent être pris en compte. Le coût et la disponibilité des appareils à ultrasons dans certains contextes peuvent également poser des difficultés. De plus, si les mesures de la VCI fournissent des informations précieuses sur l’état volémique, elles sont influencées par la variabilité individuelle, l’insuffisance tricuspidienne et les variations de la pression intra-abdominale dues à la DP 15. Il est donc essentiel d’intégrer la LUS à d’autres marqueurs.
Le temps nécessaire pour effectuer des évaluations supplémentaires lors des visites de routine est une préoccupation courante. Cependant, avec une formation adéquate, la LUS peut être réalisée rapidement, en particulier à l’aide du protocole en 8 zones, qui réduit considérablement la durée de la procédure par rapport aux méthodes traditionnelles. Compte tenu de son caractère pratique et de sa pertinence clinique, la LUS devrait être intégrée à la prise en charge liquidienne de routine des patients sous DP afin d’optimiser les soins et d’améliorer les résultats.
CONCLUSION
L’échographie pulmonaire est un outil prometteur, non invasif et hautement reproductible pour la détection précoce de la surcharge liquidienne subclinique chez les patients en DP. Elle complète efficacement d’autres méthodes, telles que l’analyse de la bioimpédance et les taux de NT-proBNP, permettant une évaluation plus complète de l’état d’hydratation, en particulier chez les patients présentant une fonction rénale résiduelle déclinante. Les résultats de cette étude doivent être interprétés avec prudence, compte tenu de la petite taille de l’échantillon, de la conception monocentrique et des critères utilisés pour définir l’hypervolémie subclinique. Dans l’ensemble, la LUS présente un potentiel pour une utilisation clinique plus large. Les perspectives d’avenir comprennent la réalisation d’études randomisées multicentriques afin de confirmer l’impact clinique de l’intégration systématique de la LUS dans le suivi des patients en DP et d’établir des protocoles standardisés pour son utilisation dans la gestion des fluides.
Contributions des auteurs
Joana Dias et Vitória Paes de Faria ont contribué à la conceptualisation et à la conception de l’étude. Elles ont collecté les données, réalisé les évaluations cliniques, effectué l’analyse des données et contribué à la rédaction du manuscrit. Ana Marta Gomes a contribué à la conceptualisation et à l’interprétation des données.
Le manuscrit a été révisé/interprété et approuvé par tous les autres auteurs : Maria Beatriz Bessa, Susana Pereira, Daniela Lopes, Rute Carmo, João Carlos Fernandes, Maria Clara Almeida et Ana Marta Gomes.
Considérations éthiques
Cette étude a été menée conformément aux principes de la Déclaration d’Helsinki. L’approbation éthique a été obtenue auprès du Comité d’éthique de l’Unidade Local de Saúde de Vila Nova de Gaia Espinho. Tous les participants ont donné leur consentement éclairé par écrit avant d’être inclus dans l’étude.
Financement
Les auteurs déclarent n'avoir reçu aucun soutien financier ni aucun financement pour la réalisation de cette étude.
Les auteurs déclarent n'avoir aucun conflit d'intérêts lié à ce travail.
Références
- Covic A., Siriopol D., Voroneanu L.. Use of Lung Ultrasound for the Assessment of Volume Status in CKD. American Journal of Kidney Diseases. 2018; 71:412-22. DOI
- Lichtenstein D., Mézière G., Biderman P., Gepner A., Barré O.. The Comet-tail Artifact. Am J Respir Crit Care Med. 1997; 156:1640-6. DOI
- Torino C., Gargani L., Sicari R., Letachowicz K., Ekart R., Fliser D.. The Agreement between Auscultation and Lung Ultrasound in Hemodialysis Patients: The LUST Study. Clinical Journal of the American Society of Nephrology. 2016; 11:2005-11. DOI
- Wang Y.K., Gargani L., Barskova T., Furst D.E., Cerinic M.M.. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: A literature review. Arthritis Res Ther. 2017; 19DOI
- Rivas-Lasarte M., Álvarez-García J., Fernández-Martínez J., Maestro A., López-López L., Solé-González E.. Lung ultrasound-guided treatment in ambulatory patients with heart failure: a randomized controlled clinical trial (LUS-HF study. Eur J Heart Fail. 2019; 21:1605-13. DOI
- Araiza-Garaygordobil D., Gopar-Nieto R., Martinez-Amezcua P., Cabello-López A., Alanis-Estrada G., Luna-Herbert A.. A randomized controlled trial of lung ultrasound-guided therapy in heart failure (CLUSTER-HF study. Am Heart J. 2020; 227:31-9. DOI
- Wang Y., Cao X., Yu J., Zhang Y., Li X., Chen X.. Association of N-Terminal Pro-brain Natriuretic Peptide With Volume Status and Cardiac Function in Hemodialysis Patients. Front Cardiovasc Med. 2021; 8DOI
- Kim Y.-L., Van Biesen W.. Fluid Overload in Peritoneal Dialysis Patients. Semin Nephrol. 2017; 37:43-53. DOI
- Baki A.H., Kamel C.R., Mansour H.. Are there any further modalities for prediction of subclinical volume overload in advanced stages of chronic kidney disease?. Kidney Res Clin Pract. 2021; 40:143-52. DOI
- Fornazarič D., Manja Antonič M., Knap B.. Volume status and arterial stiffness evaluation in peritoneal dialysis patients. Clin Nephrol. 2021; 96:74-9. DOI
- Sevinc M., Hasbal N.B., Basturk T., Ozcafer P.N., Kocas B.B., Kilickesmez K.. Comparison of lung ultrasound and other volumetric methods in peritoneal dialysis patients. Medicine. 2021; 100:e23856DOI
- Zoccali C., Torino C., Tripepi R., Tripepi G., D’Arrigo G., Postorino M.. Pulmonary Congestion Predicts Cardiac Events and Mortality in ESRD. Journal of the American Society of Nephrology. 2013; 24:639-46. DOI
- Enia G., Torino C., Panuccio V., Tripepi R., Postorino M., Aliotta R.. Asymptomatic Pulmonary Congestion and Physical Functioning in Hemodialysis Patients. Clinical Journal of the American Society of Nephrology. 2013; 8:1343-8. DOI
- Mallamaci F., Benedetto F.A., Tripepi R., Rastelli S., Castellino P., Tripepi G.. Detection of Pulmonary Congestion by Chest Ultrasound in Dialysis Patients. JACC Cardiovasc Imaging. 2010; 3:586-94. DOI
- Alexandrou M.-E., Balafa O., Sarafidis P.. Assessment of Hydration Status in Peritoneal Dialysis Patients: Validity, Prognostic Value, Strengths, and Limitations of Available Techniques. Am J Nephrol. 2020; 51:589-612. DOI
- Alexandrou M.-E., Theodorakopoulou M.P., Sarafidis P.A.. Lung Ultrasound as a Tool to Evaluate Fluid Accumulation in Dialysis Patients. Kidney Blood Press Res. 2022; 47:163-76. DOI
- Zoccali C., Torino C., Mallamaci F., Sarafidis P., Papagianni A., Ekart R.. A randomized multicenter trial on a lung ultrasound–guided treatment strategy in patients on chronic hemodialysis with high cardiovascular risk. Kidney Int. 2021; 100:1325-33. DOI
- Paudel K., Kausik T., Visser A., Ramballi C., Fan S.L.. Comparing lung ultrasound with bioimpedance spectroscopy for evaluating hydration in peritoneal dialysis patients. Nephrology. 2015; 20:1-5. DOI
- Torino C., Tripepi R., Loutradis C., Sarafidis P., Tripepi G., Mallamaci F.. Can the assessment of ultrasound lung water in haemodialysis patients be simplified?. Nephrology Dialysis Transplantation. 2021; 36:2321-6. DOI
- Buessler A., Chouihed T., Duarte K., Bassand A., Huot-Marchand M., Gottwalles Y.. Accuracy of Several Lung Ultrasound Methods for the Diagnosis of Acute Heart Failure in the ED. Chest. 2020; 157:99-110. DOI
- Kaptein M.J., Kaptein E.M.. Inferior Vena Cava Collapsibility Index: Clinical Validation and Application for Assessment of Relative Intravascular Volume. Adv Chronic Kidney Dis. 2021; 28:218-26. DOI
- Donadio C., Bozzoli L., Colombini E., Pisanu G., Ricchiuti G., Picano E.. Effective and Timely Evaluation of Pulmonary Congestion. Medicine. 2015; 94:e473DOI
- Curbelo J., Rodriguez-Cortes P., Aguilera M., Gil-Martinez P., Martín D., Suarez Fernandez C.. Comparison between inferior vena cava ultrasound, lung ultrasound, bioelectric impedance analysis, and natriuretic peptides in chronic heart failure. Curr Med Res Opin. 2019; 35:705-13. DOI
Références
1. Covic A, Siriopol D, Voroneanu L. Use of Lung Ultrasound for the Assessment of Volume Status in CKD. American Journal of Kidney Diseases 2018;71:412–22. https://doi.org/10.1053/j.ajkd.2017.10.009.
2. Lichtenstein D, Mézière G, Biderman P, Gepner A, Barré O. The Comet-tail Artifact. Am J Respir Crit Care Med 1997;156:1640–6. https://doi.org/10.1164/ajrccm.156.5.96-07096.
3. Torino C, Gargani L, Sicari R, Letachowicz K, Ekart R, Fliser D, et al. The Agreement between Auscultation and Lung Ultrasound in Hemodialysis Patients: The LUST Study. Clinical Journal of the American Society of Nephrology 2016;11:2005–11. https://doi.org/10.2215/CJN.03890416.
4. Wang YK, Gargani L, Barskova T, Furst DE, Cerinic MM. Usefulness of lung ultrasound B-lines in connective tissue disease-associated interstitial lung disease: A literature review. Arthritis Res Ther 2017;19. https://doi.org/10.1186/s13075-017-1409-7.
5. Rivas-Lasarte M, Álvarez-García J, Fernández-Martínez J, Maestro A, López-López L, Solé-González E, et al. Lung ultrasound-guided treatment in ambulatory patients with heart failure: a randomized controlled clinical trial (LUS-HF study). Eur J Heart Fail 2019;21:1605–13. https://doi.org/10.1002/ejhf.1604.
6. Araiza-Garaygordobil D, Gopar-Nieto R, Martinez-Amezcua P, Cabello-López A, Alanis-Estrada G, Luna-Herbert A, et al. A randomized controlled trial of lung ultrasound-guided therapy in heart failure (CLUSTER-HF study). Am Heart J 2020;227:31–9. https://doi.org/10.1016/j.ahj.2020.06.003.
7. Wang Y, Cao X, Yu J, Zhang Y, Li X, Chen X, et al. Association of N-Terminal Pro-brain Natriuretic Peptide With Volume Status and Cardiac Function in Hemodialysis Patients. Front Cardiovasc Med 2021;8. https://doi.org/10.3389/fcvm.2021.646402.
8. Kim Y-L, Biesen W Van. Fluid Overload in Peritoneal Dialysis Patients. Semin Nephrol 2017;37:43–53. https://doi.org/10.1016/j.semnephrol.2016.10.006.
9. Baki AH, Kamel CR, Mansour H. Are there any further modalities for prediction of subclinical volume overload in advanced stages of chronic kidney disease? Kidney Res Clin Pract 2021;40:143–52. https://doi.org/10.23876/j.krcp.20.143.
10. Fornazarič D, Manja Antonič M, Knap B. Volume status and arterial stiffness evaluation in peritoneal dialysis patients. Clin Nephrol 2021;96:74–9. https://doi.org/10.5414/CNP96S13.
11. Sevinc M, Hasbal NB, Basturk T, Ozcafer PN, Kocas BB, Kilickesmez K, et al. Comparison of lung ultrasound and other volumetric methods in peritoneal dialysis patients. Medicine 2021;100:e23856. https://doi.org/10.1097/MD.0000000000023856.
12. Zoccali C, Torino C, Tripepi R, Tripepi G, D’Arrigo G, Postorino M, et al. Pulmonary Congestion Predicts Cardiac Events and Mortality in ESRD. Journal of the American Society of Nephrology 2013;24:639–46. https://doi.org/10.1681/ASN.2012100990.
13. Enia G, Torino C, Panuccio V, Tripepi R, Postorino M, Aliotta R, et al. Asymptomatic Pulmonary Congestion and Physical Functioning in Hemodialysis Patients. Clinical Journal of the American Society of Nephrology 2013;8:1343–8. https://doi.org/10.2215/CJN.11111012.
14. Mallamaci F, Benedetto FA, Tripepi R, Rastelli S, Castellino P, Tripepi G, et al. Detection of Pulmonary Congestion by Chest Ultrasound in Dialysis Patients. JACC Cardiovasc Imaging 2010;3:586–94. https://doi.org/10.1016/j.jcmg.2010.02.005.
15. Alexandrou M-E, Balafa O, Sarafidis P. Assessment of Hydration Status in Peritoneal Dialysis Patients: Validity, Prognostic Value, Strengths, and Limitations of Available Techniques. Am J Nephrol 2020;51:589–612. https://doi.org/10.1159/000509115.
16. Alexandrou M-E, Theodorakopoulou MP, Sarafidis PA. Lung Ultrasound as a Tool to Evaluate Fluid Accumulation in Dialysis Patients. Kidney Blood Press Res 2022;47:163–76. https://doi.org/10.1159/000521691.
17. Zoccali C, Torino C, Mallamaci F, Sarafidis P, Papagianni A, Ekart R, et al. A randomized multicenter trial on a lung ultrasound–guided treatment strategy in patients on chronic hemodialysis with high cardiovascular risk. Kidney Int 2021;100:1325–33. https://doi.org/10.1016/j.kint.2021.07.024.
18. Paudel K, Kausik T, Visser A, Ramballi C, Fan SL. Comparing lung ultrasound with bioimpedance spectroscopy for evaluating hydration in peritoneal dialysis patients. Nephrology 2015;20:1–5. https://doi.org/10.1111/nep.12342.
19. Torino C, Tripepi R, Loutradis C, Sarafidis P, Tripepi G, Mallamaci F, et al. Can the assessment of ultrasound lung water in haemodialysis patients be simplified? Nephrology Dialysis Transplantation 2021;36:2321–6. https://doi.org/10.1093/ndt/gfaa285.
20. Buessler A, Chouihed T, Duarte K, Bassand A, Huot-Marchand M, Gottwalles Y, et al. Accuracy of Several Lung Ultrasound Methods for the Diagnosis of Acute Heart Failure in the ED. Chest 2020;157:99–110. https://doi.org/10.1016/j.chest.2019.07.017.
21. Kaptein MJ, Kaptein EM. Inferior Vena Cava Collapsibility Index: Clinical Validation and Application for Assessment of Relative Intravascular Volume. Adv Chronic Kidney Dis 2021;28:218–26. https://doi.org/10.1053/j.ackd.2021.02.003.
22. Donadio C, Bozzoli L, Colombini E, Pisanu G, Ricchiuti G, Picano E, et al. Effective and Timely Evaluation of Pulmonary Congestion. Medicine 2015;94:e473. https://doi.org/10.1097/MD.0000000000000473.
23. Curbelo J, Rodriguez-Cortes P, Aguilera M, Gil-Martinez P, Martín D, Suarez Fernandez C. Comparison between inferior vena cava ultrasound, lung ultrasound, bioelectric impedance analysis, and natriuretic peptides in chronic heart failure. Curr Med Res Opin 2019;35:705–13. https://doi.org/10.1080/03007995.2018.1519502.
Téléchargements
Soumis
Accepté
Publié
Comment citer
Numéro
Rubrique
Licence
© Joana Dias 2025

Ce travail est disponible sous la licence Creative Commons Attribution 4.0 International .










